(01) Draw an example of each of the four types of hydrocarbons: alkane, alkene, and alkyne.
(02) Answer True or False for the following statement.
Propane has one double bond
(03) Define the term saturation for “hydrocarbons”, then determine if the following compounds are saturated or unsaturated.
- C2H6
- C3H6
- C6H6
(04) Answer the following true and false statements accordingly.
- Double and triple bonds are capable of rotation.
- Structural isomers have the same chemical formula, but different bonding patterns.
(05) Sketch the two isomers of C4H10 and give the IUPAC name of each.
(06) Convert these IUPAC names into their skeletal structures.
- trans-2,5-dimethyl-3-octene
- 3-ethyl-5-propyloctane
- cis-1-ethyl-2-methylcyclopentane
- 4-ethyl-1-hexyne
- trans-4,5,6,7-tetramethyl-2-nonene
(07) Sketch the skeletal-line structures and chemical formulas for the following pairs of compounds to observe the differences and how even slight changes mean that compounds are structured entirely differently.
- Propane, Cyclopropane
- Ethyne, Ethene
- Cyclohexane, Benzene
- Octane, 1-Octene
- 1-pentene, cis-2-pentene
(08) Convert these IUPAC formulas into skeletal structures.
- Cyclooctane
- trans-1,3-dimethylcyclohexane
- 3,6-dimethyldecane
- cis-4-octene
- 2-hexyne
(09) For the following alkenes and alkynes, translate their IUPAC names into skeletal structures.
- cis-3-hexene
- 3-heptyne
- trans-2-butene
- 2-methyl-1-propene
- trans-3-ethyl-4-nonene
(10) Give the molecular formulas for methylcyclopentane, 2-methylpentane, and cyclohexane. Which are structural isomers?
(11) Consider the bond-line formulas shown below to answer the following questions.
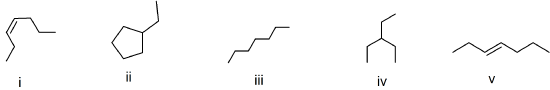
Which compounds are structural isomers? Write the chemical formula(e) to support your answer(s).
(12) Examine the following pairs of molecules. Determine whether they are identical or completely different.
(a)

(b)

(c)
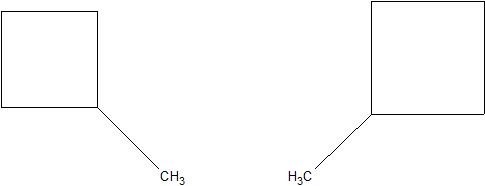
(13) Translate the following structures into their condensed formulas.
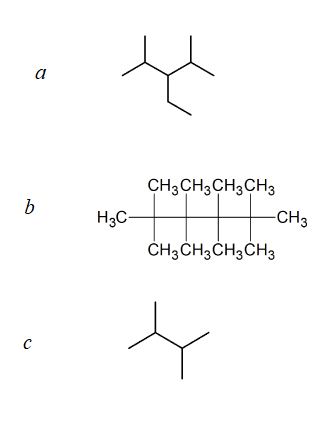
(14) Translate the skeletal structure into condensed formula and provide the IUPAC name.
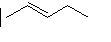
(15) Expand the given condensed structures into skeletal line structures.
a. CH3(CH2)14CH=CH2
b. CH3CH2CH(CH3)CH(CH3)CH3
c. CH3C(CH3)2CH2CH=CHCH2CH3
(16) Using your knowledge of alkane, alkene and alkyne nomenclature, name the following straight-chain molecules.
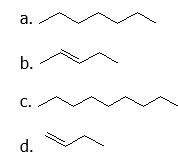
(17) Indicate whether the structures in each set represent the same compound or structural isomers.
(a)

(b)
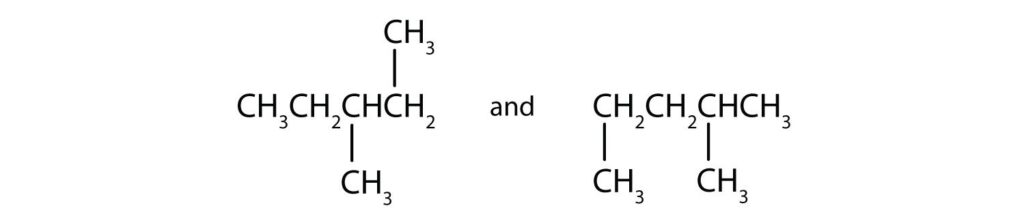
(c)

Source: Libretexts.org
