•When substances change their properties such as change of state, solubility, shape, or form, these changes are called physical changes.
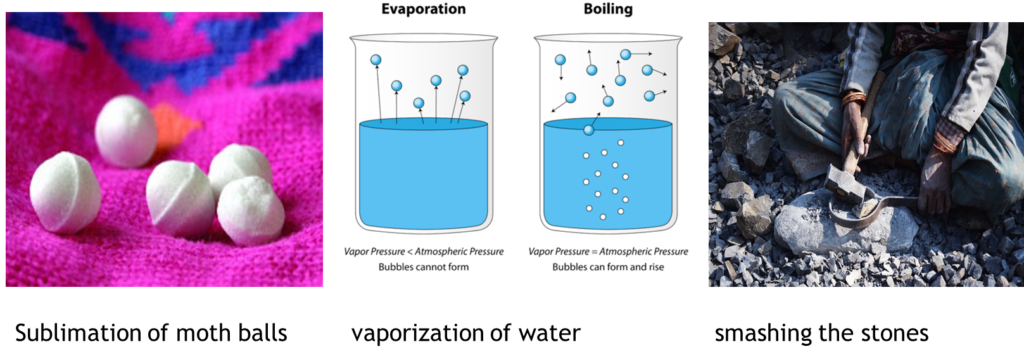
•When a new substance is formed with different compounds and properties, this change is called a chemical change.

•And, the reaction that makes this change is called a chemical reaction.
| Physical changes | Chemical changes |
| No new substances are produced | New substances are produced |
| The chemical properties of the compounds and substances remain the same although their shapes change. | New substances have entirely different chemical properties and compounds. |
| It is possibly reverted to its original form. | It is difficult to be reversed to its original form. |
Chemical Reactions
A substance before a change is called a reactant.
A substance produced after a chemical reaction is called a product.
A + B -> C + D
Reactants -> Products
2H2 (g) + O2 (g) -> 2H2O (l)
2H2O (l) -> 2H2 (g) + O2 (g)
2H2 (g) + O2 (g) -> 2H2O (l)
Hydrogen gas Oxygen gas Water
2 hydrogen molecules 1 oxygen molecule 2 water molecules
4 hydrogen atoms 2 oxygen atoms H=4 atoms, O=2 atoms
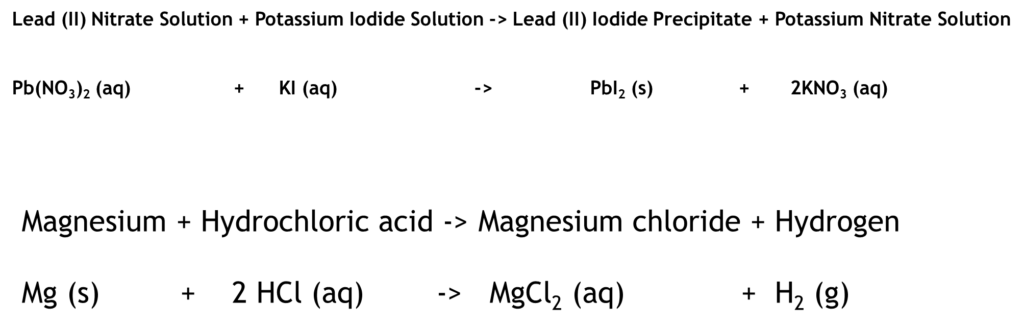
Metal + Acid -> Salt + Hydrogen
Systems and surroundings
A system refers to that part of universe in which observations are made.
The remaining part of the universe other than system is surroundings.
Types of a system:
- Open system
A system that exchanges energy or mass between the system boundary and its surroundings is an open system.
The amount of mass and energy after a change will be different.
Example: Leaving fresh limewater for a while
- Closed system
A closed system is a system that exchanges only energy.
No mass transfer occurs between the system and its surroundings.
Therefore the amount of mass after a change remains the same but the energy changes.
Examples: Solubility of sugar in water and melting ice.
- Isolated system
A system that can’t transfer both energy and mass outside the system boundary is an isolated system.
Example: thermally insulated bottles
Law of conservation of mass
“Mass in any closed or isolated system can neither be created nor destroyed by chemical reactions and physical transformations” – Antoine Laurant Lavoisier
Closed system
A + B -> C + D
Mass A + Mass B = Mass C + Mass D
2Mg + O2 -> 2MgO
Mass Mg + Mass O2 -> Mass MgO
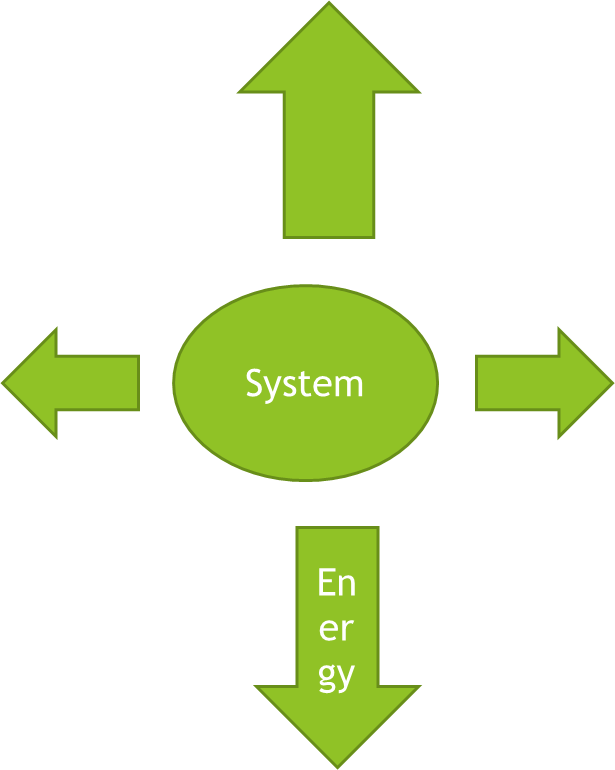
Exothermic Change
When there is high energy in a system after a change, it means there is a release of heat, which raises the temperature of its surroundings; this change is called exothermic change.
Examples: solubility of calcium chloride, combustion of fuel, breathing, condensation of water vapour
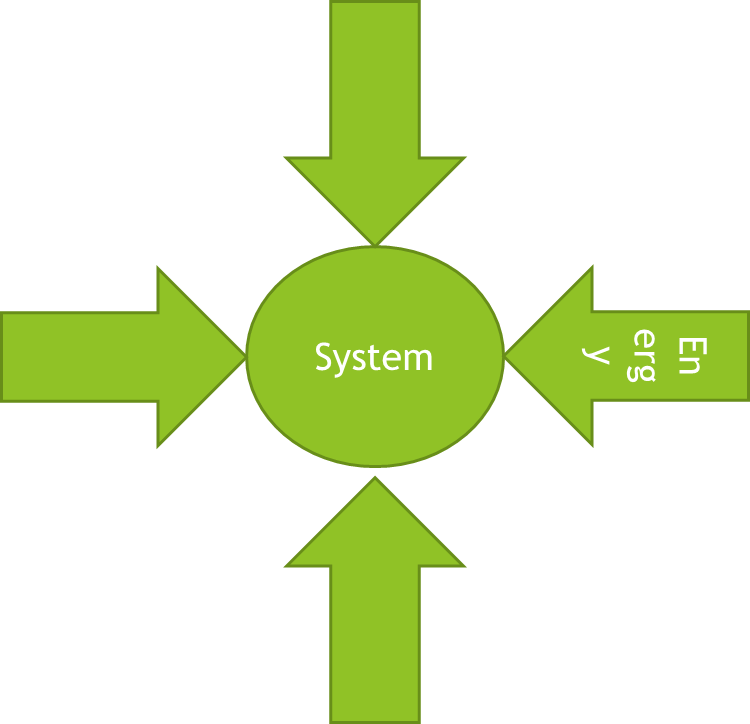
Endothermic Change
When a temperature is lower after a change, it means there is an absorption of heat from the surroundings.
This lowers its temperature.
This change is called endothermic change.
Examples: evaporation of sweat, the solubility of potassium nitrate, melting of ice, etc.
Combustion
Combustion is a chemical reaction that occurs when a fuel, which mostly consists of elements, carbon (C) and/or hydrogen (H)reacts with oxygen.
This compound of Carbon and Hydrogen is known as a hydrocarbon.
Incomplete Combustion
Incomplete combustion occurs when there is limited oxygen supply and/or heat energy.
Carbon soot and water are formed.
Fuel + Oxygen -> Carbon monoxide + Soot + Water Vapour
Deficient Carbon
C3H8 (g) + 3O2 (g) -> 2CO (g) + C (s) + 4H2O (g)
Propane Oxygen Carbon monoxide Carbon Water Vapour
Harmful Effects

How to prevent it?
- taking the public transformation to reduce fuel combustion.
- vehicle inspection on a regular period.
Experimental Skills
Using and organizing techniques, apparatus, and materials
Balance
* The unit of mass is the kilogram (kg).
* 1 kg = 1000 grams
• A beam balance is accurate to the size of the smallest mass that tilts the balance beam.
• A digital top-pan balance is accurate to the size of the smallest mass that can be measured on the scale setting you are using, probably 0.01 g.
• When using an electronic balance you should wait until the reading is steady before taking it.
Measuring Cylinder
• The volume of a liquid can be obtained by pouring it into a measuring cylinder.
• Measuring cylinders are often marked in milliliters (ml) where 1 milliliter = 1 cm3
• The accuracy of the reading will be 1 cm3
• Note that 1 litre= 1000cm3 = 1 dm3
1000 millilitres = 1000 cm3
