Hydrocarbons
• Carbon atoms bond to each other by single, double, and triple bonds.
• Saturated hydrocarbons contain only single bonds.
• Unsaturated hydrocarbons contain at least one double or triple bond.
Refining Hydrocarbons
• Fractional distillation involves boiling petroleum and collecting each group of components as they condense at different temperatures.
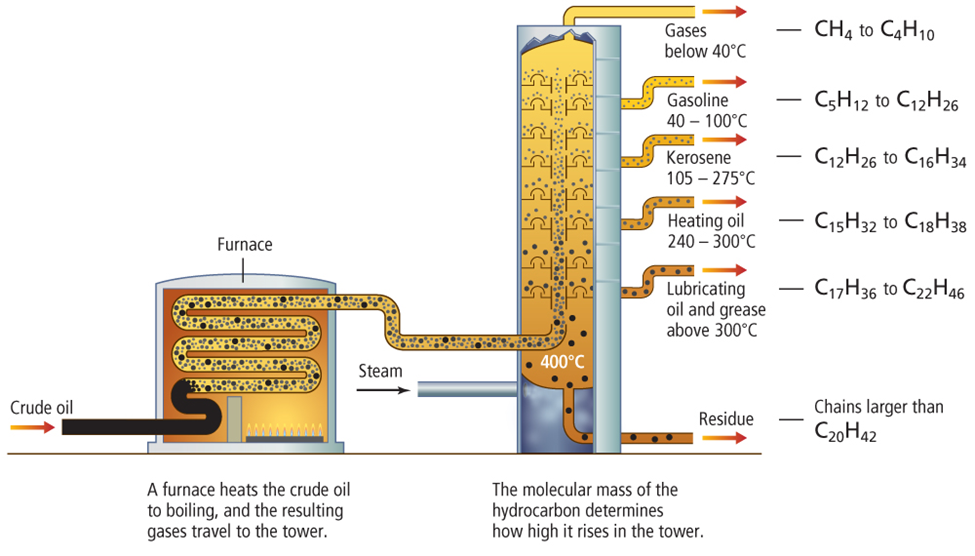
• Fractional distillation towers do not yield fractions in proportion to demand.
• Heavier fractions are converted to gasoline or other lighter fractions by a process called cracking.
*Cracking is done in the absence of oxygen and in the presence of a catalyst.
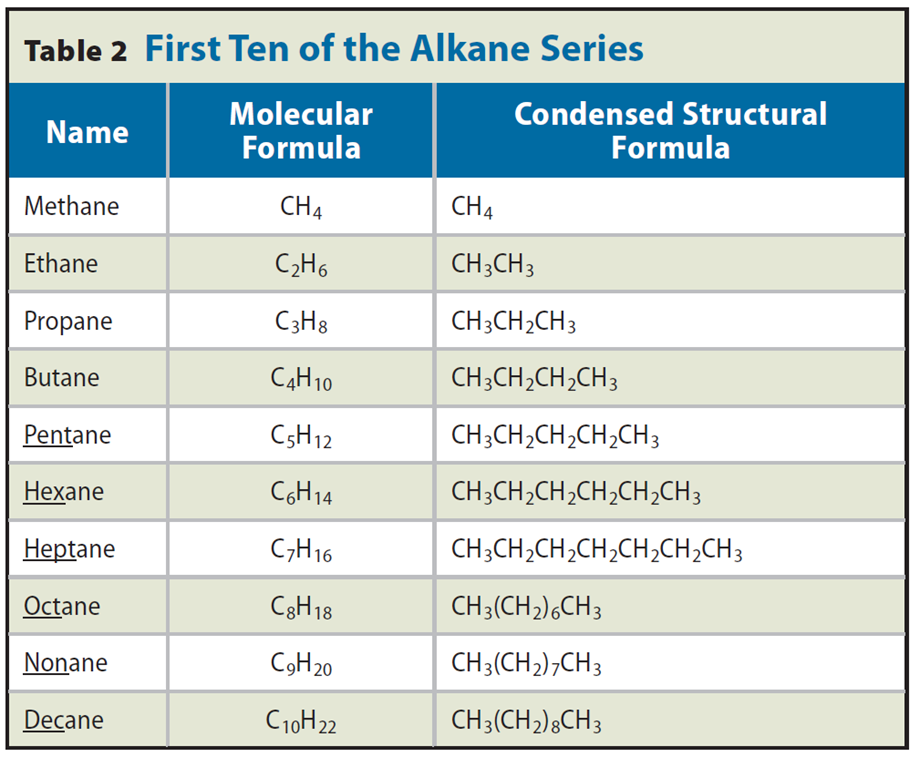
Straight-Chain Alkanes
• The names of alkanes end in –ane.
• Prefixes are derived from Greek numbers.
• A series of compounds that differ from one another by a repeating unit is called a homologous series.
• For alkanes, the relationship between the numbers of carbon and hydrogen atoms can be expressed as: CnH2n + 2
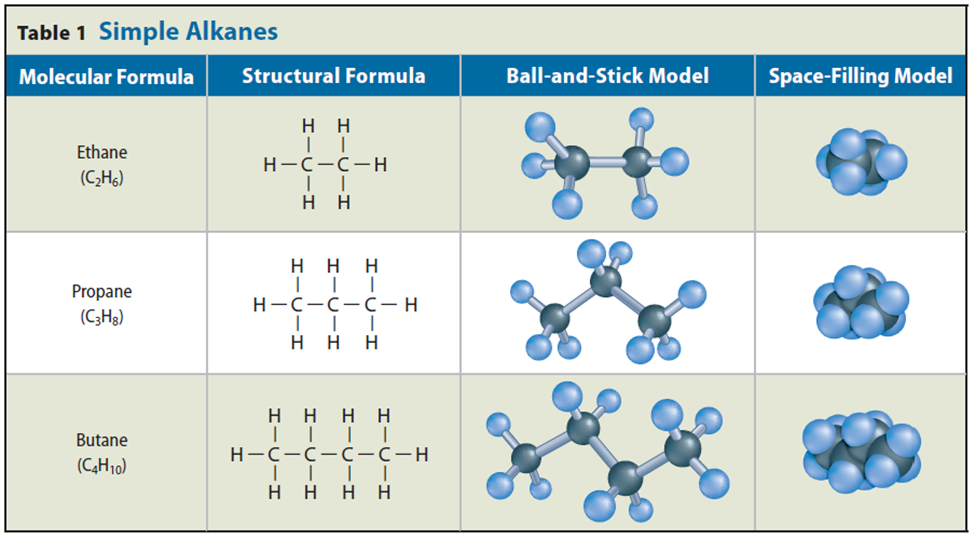
Straight-Chain Alkanes
• Alkanes are hydrocarbons that have only single bonds between atoms.
Branched-Chain Alkanes
• Naming branched-chain alkanes
– Count the number of carbon atoms in the longest continuous chain.
– Number each carbon in the parent chain, starting with the carbon closest to the substituent group. This gives all the substituent groups the lowest position numbers possible.
– Name each alkyl group substituent.
– If the same alkyl group appears more than once as a branch on the parent structure, use a prefix to indicate how many times it appears.
– When different alkyl groups are attached to the same parent chain, place their names in alphabetical order.
– Write the entire name, using hyphens to separate numbers from words and commas to separate numbers.
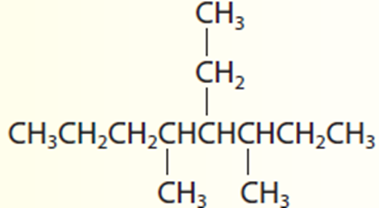
4-ethyl-3,5-dimethyloctane.
Cycloalkanes
• An organic compound that contains a hydrocarbon ring is called a cyclic hydrocarbon.
• Cyclic hydrocarbons with only single bonds are called cycloalkanes.
• The relationship between numbers of carbon and hydrogen atoms in cycloalkanes can be expressed as: CnH2n

Cycloalkanes
• Naming substituted cycloalkanes is the same as straight-chains but with a few exceptions.
– The ring is always considered the parent chain.
– Numbering starts on the carbon that is bonded to the substituent.
– When more than one carbon has a substituent, number in the direction that gives the lowest possible numbers for the substituents.
• Alkenes
• Unsaturated hydrocarbons that contain at least one or more double covalent bonds between carbon atoms are called alkenes.
Alkenes
• Alkenes are named in much the same way as alkanes.
• Alkenes end in –ene.
• When four or more carbon atoms are present, specify the location of the double bond.
• When naming branched-chain alkenes, follow the same rules as for alkanes, with two exceptions.
• The parent chain is always the longest chain that contains double bond, whether it is the longest chain or not.
• The position of the double bond, not the branches, determine the numbering.
• Use a prefix to designate the number of double bonds.
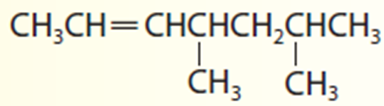
4,6-dimethyl-2-heptene.
Alkynes
• Unsaturated hydrocarbons that contain one or more triple bonds between carbon atoms are called alkynes.
• Straight-chain and branched-chain alkynes are named in the same way as alkenes, except the ending is –yne.
• Isomers are two or more compounds that have the same molecular formula but different molecular structures.
• Structural isomers have the same chemical formula but their atoms are bonded in different arrangements.
Stereoisomers
• Geometric isomers result from different arrangements of groups around a double bond.
• Cis means on the same side, and trans means across from.
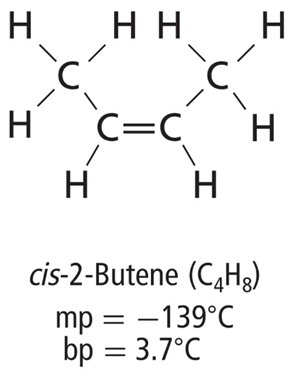
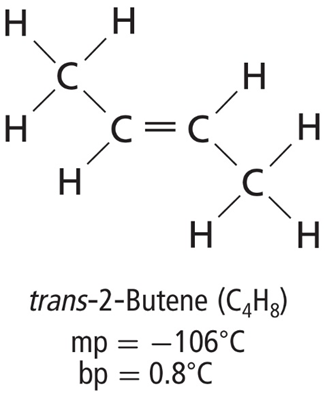
• The property in which a molecule exists in right and left-handed forms is called chirality.
• Organic compounds that contain benzene rings as part of their structure are called aromatic compounds.
• Aromatic was originally used because many benzene-related compounds were found in pleasant-smelling oils that came from plants and plant parts.
• Aliphatic compounds are the alkane, alkene, and alkyne hydrocarbons, coming from the Greek word for fat because they were obtained by heating animal fat.
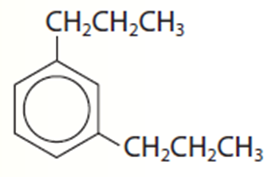
1,3-dipropylbenzene.
• Substituted benzene compounds are named in the same way as cyclic alkanes.
Organic Compounds Containing Halogens
• Any organic compound that contains a halogen substituent is called a halocarbon.
• An alkyl halide is an organic compound containing a halogen atom covalently bonded to an aliphatic carbon atom.
• An aryl halide is an organic compound containing a halogen bonded to an aromatic group.
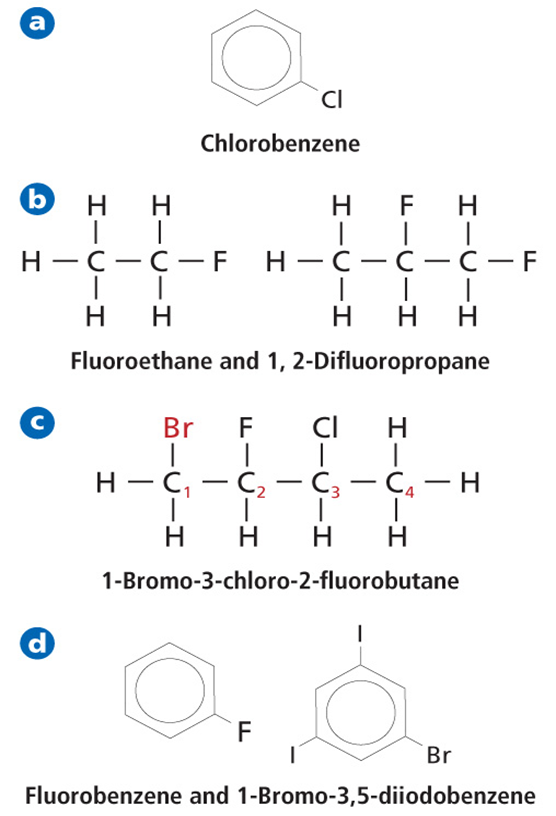
• Organic molecules containing functional groups are given IUPAC names based on their main chain alkane structures.
‒ For alkyl halides, a prefix indicating the halogen as follows: remove ine and add o. Ex. Fluoro, chloro, bromo, etc.
‒ If there is more than a single halogen, they are listed alphabetically.
• A substitution reaction is one in which one atom or a group of atoms in a molecule is replaced by another atom or group of atoms.
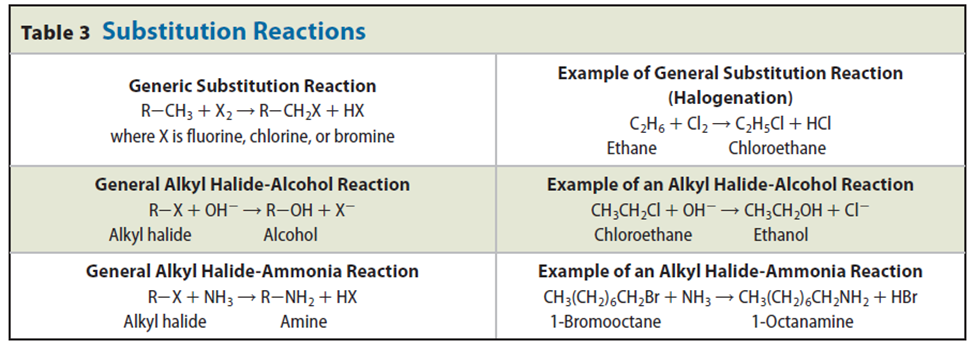
Hydrogen atoms in an alkane can be replaced by atoms of halogens in a process called halogenation.
Classifying Reactions of Organic Substances
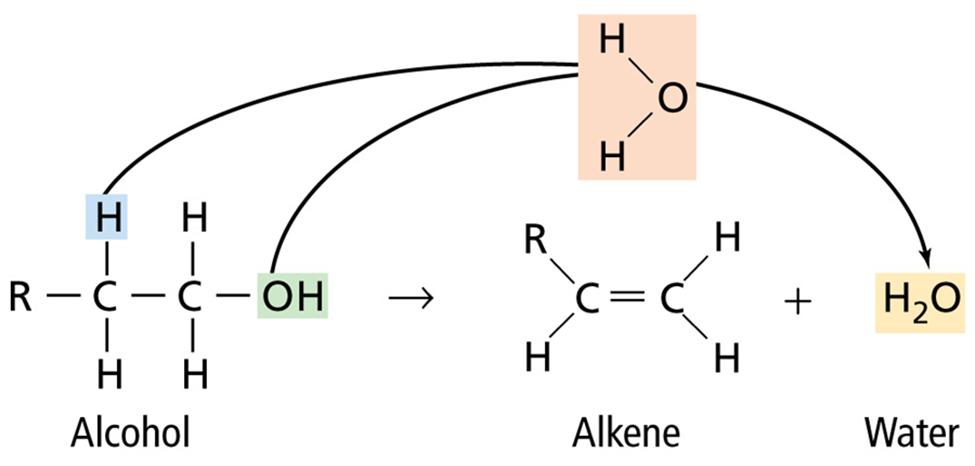
• An elimination reaction in which the atoms removed form water is called a dehydration reaction.
• Denatured alcohol is ethanol with small amounts of noxious materials added to it.
• Alcohol names are based on the alkane names, with the ending –ol.
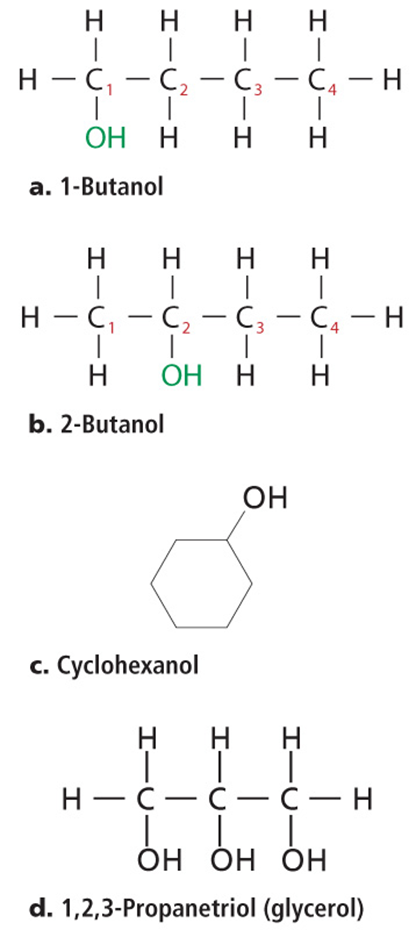
Organic Compounds Derived from Carboxylic Acids
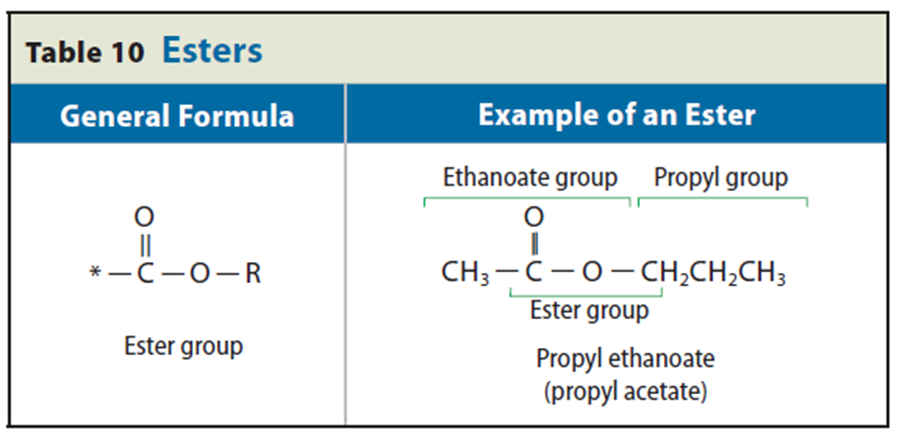
• An ester is any organic compound with a carboxyl group in which the hydrogen in the hydroxyl group is replaced by an alkyl chain.
• To name an ester, write the alkyl group followed by the name of the acid with the –oic acid ending replaced with –oate.
Condensation Reactions
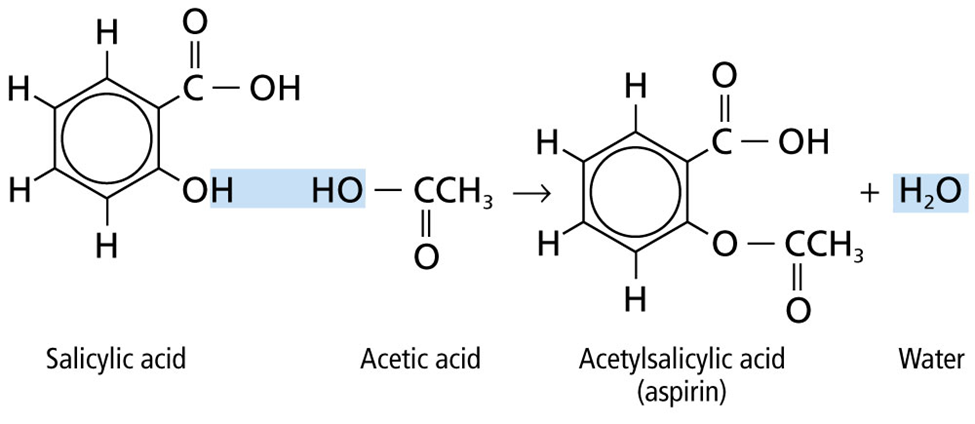
• In a condensation reaction, two smaller organic molecules combine to form a more complex molecule, accompanied by the loss of a small molecule such as water.
• Condensation reactions are elimination reactions that form bonds between two atoms not previously bonded.
Disposal of Plastics and its useful and harmful effects.
Combustion of Alcohol:
C2H5OH + 3O2 -> 2CO2 + 3H2O
Clean Blue Flame
Substitution (Reaction of Alcohol with Hydrogen Halide):
CH3CH2OH + HCl -> CH3CH2Cl + H2O
Dry HCl can be prepared by:
NaCl + H2SO4 -> NaHSO4 + HCl
Esterification:
Reaction that involves the breaking of O-H bond in alcohols is known as Esterification.
Carboxylic acid + alcohol -> ester + Water (in presence of acid catalyst)
CH3COOH + C2H5OH -> CH3COOC2H5 + H2O (in presence of H2SO4)
Ethanoic acid + ethanol -> ethyl ethanoate + water
C2H5COOH + C2H5OH -> C2H5COOC2H5 + H2O (in presence of H2SO4)
Propanoic acid ethanol ethyl propanoate water
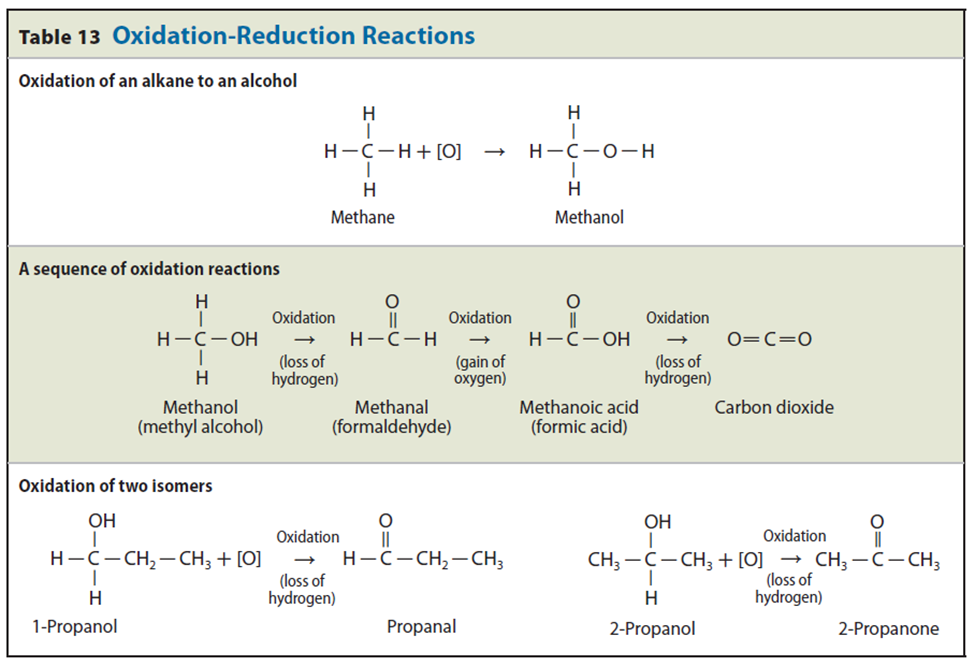
Alkanes are saturated hydrocarbons with a central carbon atom attached to four other atoms (or groups). This saturation leads to relatively low reactivity of alkanes.
Traditionally in old times, saturated hydrocarbons were known as paraffins meaning “little affinity”. However, these alkanes burn very rapidly.
The combination of alkanes with oxygen-generating heat is known as combustion.
More precisely, combustion is defined as “a chemical reaction with oxygen in which alkane is converted into carbon dioxide and water with the release of heat energy”.
Equation 1: Combustion of methane
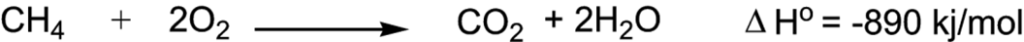
Source: McGraw Hill Education

Comments
Appreciate this post. Will try it out.