Atoms consist of three basic particles: protons, electrons, and neutrons.
The nucleus (center) of the atom contains the protons (positively charged) and the neutrons (no charge).
The outermost regions of the atom are called electron shells and contain electrons (negatively charged).
- mass number: The sum of the number of protons and the number of neutrons in an atom.
- atomic number: The number of protons in an atom.
Mass Number
An element’s mass number (A) is the sum of the number of protons and the number of neutrons. The small contribution of mass from electrons is disregarded in calculating the mass number.
Atomic Number
Neutral atoms of an element contain an equal number of protons and electrons. The number of protons determines an element’s atomic number (Z) and distinguishes one element from another.
Electronic structure
An electronic structure is the way in which electrons are arranged in an atom.
Electrons in shells
Electrons in atoms occupy energy levels, also called electron shells, outside the nucleus. Different shells can hold different maximum numbers of electrons. The electrons in an atom occupy the lowest available energy level first. This is the shell nearest the nucleus. When this shell is full the electrons begin to occupy the next energy level.
Below is a table showing the maximum number of electrons an element can have for each of its energy level shells. The information shown is for elements with atomic numbers 1 to 20:
| Shell | Maximum |
|---|---|
| First | 2 |
| Second | 8 |
| Third | 8 |
Predicting an electronic structure
The electronic structure of an atom can be predicted from its atomic number. For example, the atomic number of sodium is 11. Sodium atoms have 11 protons and so 11 electrons:
- two electrons occupy the first shell
- eight electrons occupy the second shell
- one electron occupies the third shell
This electronic structure can be written as 2,8,1 (each comma, or dot, separates one shell from the next). This electronic structure can also be shown as a diagram. In these diagrams:
- each shell is shown as a circle
- each electron is shown as a dot or a cross
The electronic structure of sodium as a diagram
Electronic structures and the periodic table
The electronic structure of an element is linked to its position on the periodic table.
| Electronic structure feature | Link to the periodic table |
|---|---|
| Number of shells | Period number |
| Number of electrons in the outermost shell | Group number |
Endothermic Reactions
The endothermic process is a term that describes a reaction where the system absorbs the energy from its surrounding in the form of heat. A few examples of the endothermic process are photosynthesis, evaporating liquids, melting ice, dry ice, alkanes cracking, thermal decomposition, ammonium chloride in water and much more.
Exothermic Reactions
The exothermic reaction is the opposite of an endothermic reaction. It releases energy by light or heat to its surrounding. A few examples are neutralization, burning a substance, reactions of fuels, deposition of dry ice, respiration, solution of sulfuric acid into water and much more.
Exothermic reactions transfer energy to the surroundings and the temperature of the surroundings increases.
Endothermic reactions take in energy and the temperature of the surroundings decreases.
Group 1 – the alkali metals
The Group 1 elements in the periodic table are known as the alkali metals. They include lithium, sodium and potassium, which all react vigorously with water to produce an alkaline solution.
Group 1 – the alkali metals
The Group 1 elements are called the alkali metals. They are placed in the vertical column on the left-hand side of the periodic table.
All the Group 1 elements are very reactive. They must be stored under oil to keep air and water away from them. Group 1 elements form alkaline solutions when they react with water, which is why they are called alkali metals.
The reactivity of Group 1 elements increases as you go down the group.
Group 7 – the halogens
The Group 7 elements are called the halogens. They are placed in the vertical column, second from the right, in the periodic table.
Chlorine, bromine and iodine are the three common Group 7 elements. Group 7 elements form salts when they react with metals. The term ‘halogen’ means ‘salt former’.
Group 8A (or VIIIA) of the periodic table are the noble gases or inert gases: helium (He), neon (Ne), argon (Ar), krypton (Kr), xenon (Xe), and radon (Rn). The name comes from the fact that these elements are virtually unreactive towards other elements or compounds.
- Combustion is another name for burning.
- In a combustion reaction, fuel is burned and reacts with oxygen to release energy.
Rate of Reaction
Particles can only react when they collide. If you heat a substance, the particles move faster and so collide more frequently. That will speed up the rate of reaction.
When more particles are present in a given amount of space, a greater number of collisions will naturally occur between those particles. Since the rate of a reaction is dependent on the number of collisions occurring between reactants, the rate increases as the concentration increases.
If the surface area of a reactant is increased: more particles are exposed to the other reactant. there is a greater chance of particles colliding, which leads to more successful collisions per second.
A chart of the reactivity series of common metals is provided below.
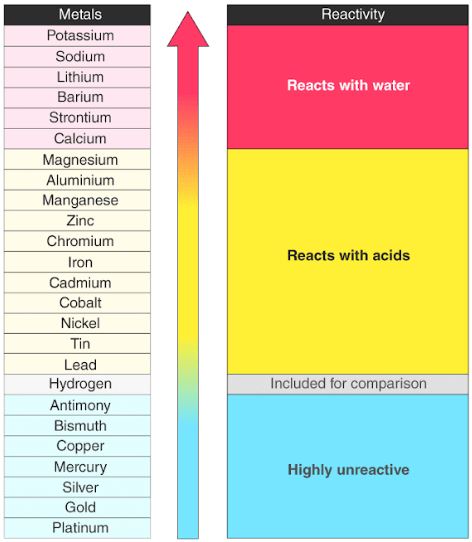
Reaction of Metals
When a metal reacts with oxygen, a metal oxide forms. The general equation for this reaction is:
metal + oxygen → metal oxide.
Rust is a form of iron oxide and it forms slowly when iron is exposed to air.
Metal + acid → salt + hydrogen
When a metal is put in acid, it gets smaller and smaller as it gets used up in the chemical reaction . At the same time, bubbles of gas can be seen. The bubbles produced in the reaction are hydrogen gas. This can be proven using a burning splint because hydrogen is flammable. When the burning splint is put into the test tube containing hydrogen gas, a small explosion occurs, making a squeaky pop sound. This shows that hydrogen is present.
Displacement reactions involve a metal and the compound of a different metal. A more reactive metal will displace or push out a less reactive metal from its compound in a displacement reaction. The less reactive metal is left uncombined after the reaction. It is no longer chemically bonded to any other elements. It is now a pure element.
Example
Magnesium is more reactive than copper. When a piece of magnesium is dipped into blue copper sulfate solution, a displacement reaction occurs.
The magnesium displaces the copper, and the products are copper and a solution of magnesium sulfate.
This is the word equation:
magnesium + copper sulfate → copper + magnesium sulfate
Reactions of Acids and Bases
When an acid and a base react, the reaction is called a neutralization reaction. That’s because the reaction produces neutral products. Water is always one product, and a salt is also produced. A salt is a neutral ionic compound.
Let’s see how a neutralization reaction produces both water and a salt, using as an example the reaction between solutions of hydrochloric acid and sodium hydroxide. The overall equation for this reaction is:
NaOH + HCl → H2O and NaCl
Acid reactions with carbonates and hydrogencarbonates
Acids react with metal carbonates and hydrogencarbonates in the same way. These reactions produce salt, water and carbon dioxide.
acid + carbonate → salt + water + carbon dioxide
or
acid + hydrogencarbonate → salt + water + carbon dioxide
Example – carbonate:
hydrochloric acid + copper(II) carbonate → copper(II) chloride + water + carbon dioxide
2HCl(aq) + CuCO3(s) → CuCl2(aq) + H2O(l) + CO2(g)
Observations: green solid copper(II) carbonate disappears, blue solution produced, heat released, bubbles.
Example – hydrogencarbonate:
hydrochloric acid + sodium hydrogencarbonate → sodium chloride + water + carbon dioxide
HCl(aq) + NaHCO3 (s) → NaCl(aq) + H2O(l) + CO2(g)
Observations: solid white sodium hydrogencarbonate disappears, colourless solution produced, bubbles.
The carbon dioxide gas produced in these reactions can be tested. The test for carbon dioxide is:
- bubble gas into colourless limewater (calcium hydroxide solution)
- the solution will change from colourless to milky if the gas is carbon dioxide
References:
https://www.bbc.co.uk/bitesize/guides/zg923k7/revision/3
https://www.bbc.co.uk/bitesize/guides/zmjyqp3/revision/6
https://www.bbc.co.uk/bitesize/topics/zypsgk7/articles/z9sptrd
https://www.bbc.co.uk/bitesize/topics/zn6hvcw/articles/zvfxxbk
https://www.bbc.co.uk/bitesize/topics/zypsgk7/articles/zcwxcj6
https://byjus.com/chemistry/reactivity-series/
https://www.chemguide.co.uk/physical/basicrates/temperature.html
https://www.angelo.edu/faculty/kboudrea/periodic/periodic_main8.htm
https://byjus.com/chemistry/endothermic-exothermic-reactions-difference/
https://www.bbc.co.uk/bitesize/guides/z2b2k2p/revision/2
https://www.bbc.co.uk/bitesize/guides/z3xn82p/revision/1
